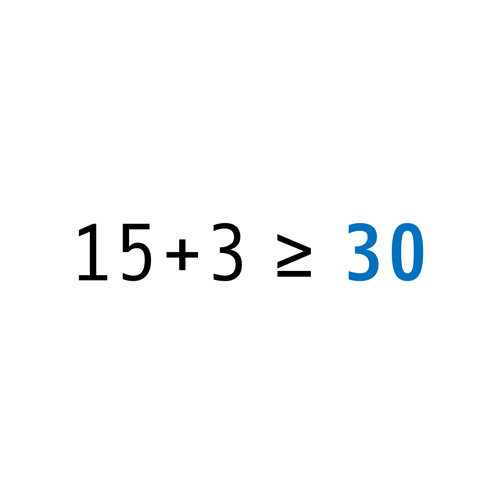gempex is 20!
The excitement is high when you found a company. Where the journey leads and its success remain uncertain. You are highly motivated, confident in yourself and your idea, ready to do whatever it takes to reach far-off and ambitious goals.
But it’s not always easy. The journey is marked by success as well as failures. On the way, you soon learn that having the right goal is important, but those who accompany you on the journey matter even more.
The goal – being the GMP expert – turned out to be the right one on our journey, albeit very challenging as well. Quality, reliability, ongoing learning, openness to new things – these values have defined the gempex journey, associating the company’s name with those attributes in the market. Yet quality does not mean freedom from mistakes. Like the maintenance of a ship on its journey, identifying, admitting and eliminating mistakes translates into ongoing upkeep and improvement.
However, the best goal is of little use unless you have people on board striving for the same goal, with equal motivation and dedication. Employees and also our customers have been part of the long and successful journey that defined gempex as a trademark. More than 80 GMP experts, distributed over several offices in three key countries that define the market, working for nearly all sectors of the life sciences industry, from manufacturing to supply, bear witness to a lasting, now well established and widely known enterprise.
I would like to thank everyone – customers, friends, business partners and especially all employees – who made this success happen!
Have we reached our goal, finished our journey? By no means! The goal has become more tangible, we are closer to it, yet it continues to pose new challenges. Over the coming years, gempex will rise to these challenges and I appreciate everyone who will be part of this ongoing, exciting and turbulent journey.
With kind regards
Ralf Gengenbach
Founder and Managing Director
gempex GmbH - THE GMP-EXPERT