gempex – THE GMP-EXPERT
Willkommen!
gempex - Expertise in Life Sciences
Eckpunkte der Unternehmensentwicklung
GMP ist in der Life Sciences Industrie der Schlüssel zu Märkten und Markterfolg.




Willkommen!
gempex - Expertise in Life Sciences
Eckpunkte der Unternehmensentwicklung
GMP ist in der Life Sciences Industrie der Schlüssel zu Märkten und Markterfolg.
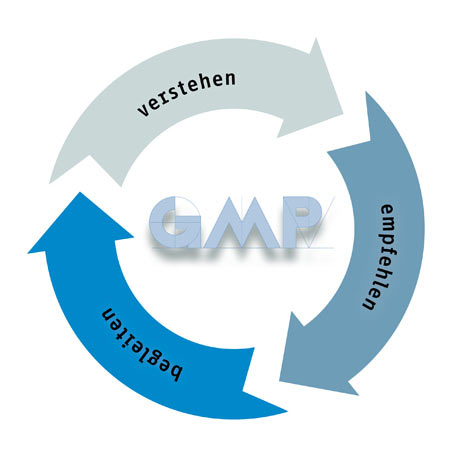
Als international tätiger GMP-Experte bietet gempex neben der erforderlichen Qualität auch die notwendige Sicherheit für alle Fragen der Compliance. gempex genießt das Vertrauen der Kunden. Weil gempex Situationen und Aufgaben versteht, fundierte und pragmatische Empfehlungen ausspricht und Kunden in der Umsetzung begleitet.
Das Dienstleistungsspektrum der gempex umfasst die Bereiche GMP-Compliance, Commissioning & Qualifizierung, IT-Validierung und GMP-Routine. Abhängig von der Aufgabenstellung und den persönlichen Bedürfnissen erfolgt die Unterstützung in Form von Beratung, Projektabwicklung, Onsite Execution. GMP Consulting & Execution.
Das versteht gempex unter „Full Service“ für Compliance-Aufgaben. Ob Kunden nur das Wissen benötigen, die Aufgabe als Projekt umsetzen lassen möchten oder die Experten temporär ins Haus holen wollen. Auch alle Formen der Unterstützung gemeinsam sind möglich – bedarfsgerecht und flexibel.
gempex genießt als GMP-Experte internationales Ansehen und unterstützt auf dem Weg zu sicherer, effizienter Compliance mit den Good Practices, vergleichbaren Qualitätssystemen und anzuwendenden Regularien. Inspection Readiness für die Industrien Pharma, Chemie, Biotech, Medizinprodukte, Kosmetik und deren Lieferanten.
gempex – THE GMP-EXPERT